Sodium-ion batteries (SIBs) tend to show higher thermal stability, slower temperature rise during abuse, reduced gas/flammable-byproduct generation, and improved transport safety compared with many lithium-ion chemistries. Those advantages arise from differences in electrode chemistry (materials and reaction pathways), electrolyte behavior, and the way the solid-electrolyte interphase (SEI) forms and breaks down during abuse. Real-world tests and reviews show SIBs are not immune to hazards but generally have lower likelihood and reduced severity of thermal runaway than many lithium chemistries used today — especially high-energy NCM packs.
1) What we mean by “safer”
Battery safety covers many things — thermal runaway (an exothermic chain reaction producing heat, fire, or explosion), flammability of electrolytes, gas evolution under abuse, dendrite formation leading to short circuits, and transport/handling hazards. When people say “safer”, they usually mean a combination of:
- Higher onset temperature for thermal runaway (takes more heat to go unstable),
- Slower temperature rise if something starts to fail (buying time for protection),
- Less flammable gases / lower heat release during an event, and
- Lower risk during transport and mechanical abuse (puncture, crush, overcharge).
2) The chemistry differences that matter (simple map)
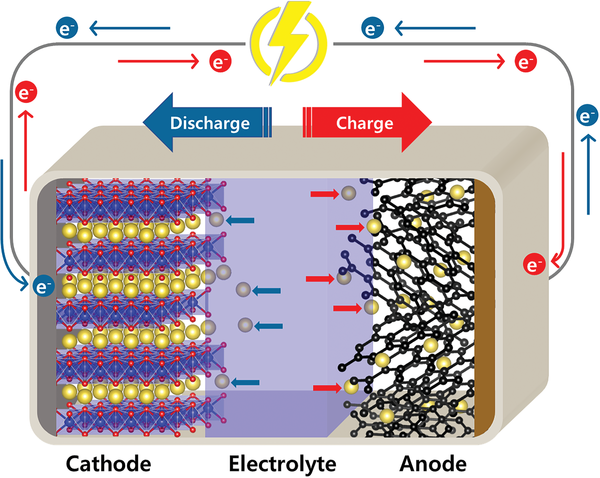
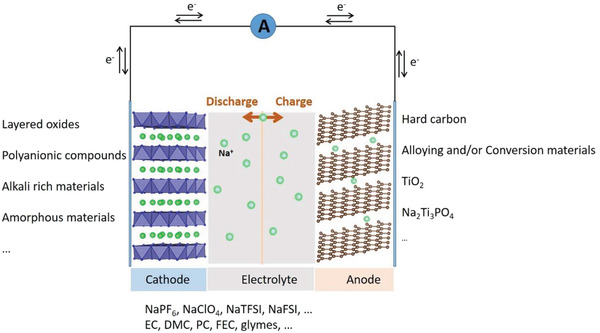
Think of a battery cell as three key layers: cathode — electrolyte — anode. Differences in these components determine how energy and heat are produced or released during abuse.
- Cathode chemistry: Many SIBs use layered oxides or polyanionic cathodes whose decomposition pathways produce fewer highly-reactive gases than some high-energy lithium cathodes (e.g., NCM/NCA). That tends to reduce exothermic chain reactions.
- Anode chemistry: SIBs commonly use hard carbon, not graphite. Hard carbon forms an SEI that behaves differently under heat and can be more robust in some formulations (less violent SEI breakdown). Lithium metal or graphite can generate worse SEI breakdown and gas when overcharged or heated.
- Electrolyte & salts: SIB electrolytes often use sodium salts (NaPF₆, NaFSI) in carbonate solvents — and there’s active development of non-flammable electrolytes (ionic liquids, flame-retardant additives, aqueous or quasi-aqueous electrolytes) specifically for SIBs that lower flammability risk further.
These material differences alter how and when heat-producing reactions start and whether they cascade into thermal runaway.
3) Experimental evidence: thermal-runaway behavior and metrics

Recent comparative tests and review papers report consistent trends:
- Onset temperature: Studies find sodium-ion cells often exhibit thermal-runaway onset at similar or slightly higher temperatures than many lithium chemistries (and notably higher than certain high-energy NCM cells). Some reviewers place SIB onset and severity between LFP (very safe) and NCM (higher risk).
- Rate of temperature rise: Abuse tests show SIBs usually heat up more slowly once a failure begins (e.g., temperature rise rates several times lower than comparable LIBs in the same conditions). That gives BMS/EMS and thermal management more time to react.
- Gas & heat release: Some tests measure less total gas and less energetic decomposition for SIB cathode/electrolyte systems relative to NCM-based LIBs; LFP still often scores best on this metric.
Bottom line from experimental literature: SIBs reduce both the probability and the speed/severity of catastrophic events versus many LIB types, though they are not risk-free.
4) Why the differences reduce fire/explosion risk — the mechanisms (plain English)
- Less reactive decomposition pathways: when heated, SIB electrode materials tend to decompose in ways that release less flammable gas and less heat per gram than some lithium counterparts, so once abuse begins it’s less likely to cascade into full runaway.
- SEI stability: the SEI (thin passivation layer) formed on hard carbon anodes in SIBs tends to break down less violently than graphite SEI in some Li chemistries, so short-circuit triggers are less energetic.
- Electrolyte innovations: SIB research actively pursues non-flammable or flame-retardant electrolytes and safer salts that reduce flammable vapor formation during abuse. These choices can further tip safety in SIBs’ favor.
- Transport & SOC handling: certain SIB pack designs can be shipped at zero volt (or near-zero SOC) more readily than commercial LIBs; shipping at zero SOC reduces transport fire risk. Industry players highlight this as a real logistic safety advantage.
5) Real-world implications for ESS (energy storage systems)
- Grid & residential ESS: For stationary systems where energy density is less important, SIBs’ safety profile (lower thermal runaway risk, wider operating temperature range in some products) is a strong asset. That reduces HVAC/heating requirements and can simplify pack designs.
- Transport and storage logistics: SIBs offer safer handling and transport advantages (lower SOC shipping options and reduced regulatory burden), lowering total system risk for installers and integrators.
- Integration with BMS/EMS: even with safer chemistries you must still design robust BMS, ventilation, and fire-suppression strategies. Safer chemistry is not a substitute for good engineering.
6) Practical checklist for a safety-focused ESS spec (what to ask suppliers)
When evaluating SIB products specifically for safety, request:
- Independent abuse test reports (thermal runaway, nail/penetration, overcharge).
- Onset temperature and heat-release rate measurements vs LFP/NCM benchmarks.
- Electrolyte composition (presence of flame retardants / non-flammable formulations).
- Transport state recommendations (can they ship at zero-V / zero SOC?).
- Long-term cycle tests for SEI stability and calendar life under local environmental extremes.
7) Quick comparative snapshot
8) Recommended reading (key sources used)
- Comparative thermal-runaway and thermal stability reviews for sodium vs lithium. MDPI+1
- Recent experimental abuse tests and industry summaries (ESS-News comparison, IDTechEx). Energy Storage+1
- Reviews on non-flammable electrolytes and SEI behavior for sodium systems. RSC Publishing+1
9) Bottom line (for your ESS business)
Sodium-ion batteries offer a meaningful safety advantage over many lithium-ion chemistries used today — mainly via higher thermal stability, slower failure progression, and options for less-flammable electrolytes and safer transport. For grid and stationary ESS where size/weight are secondary, that safety edge can reduce balance-of-system costs (cooling, containment, insurance) and simplify regulatory hurdles. But don’t treat it as a free pass — test reports, BMS design, and good packaging remain essential.
